Analysis Of Glucose Molecules
Question
Task: What is Glucose molecule? Explain its regulations and functions.
Answer
The word glucose came into existence from a Greek word glukus which refers to sweet. A scientist from Germany in 1747 named as Andreas Marggraf isolated the glucose from raisins. Another scientist, Johann Lowitz discovered that grapes have a glucose which is different than regular sugarcane. Later in 1883, the term glucose was given by Jean Dumus (Barclay, Cooper, Ginic-Markovic & Petrovsky, 2010). The present study will help in understanding about Glucose molecules, its regulation and functions.
Glucose molecule is also a monosaccharide and the latter is also termed as simple sugar. It is one among the three monosaccharide’s which is being used by our body. It is considered in biology as one of the important carbohydrates. It directly helps in the production of Adenosine triphosphate (ATP). In order to produce energy in the body, ATP is used. It is the only single molecule which can be used to produce energy. Therefore, the required level of glucose molecule is essential for the body.
Glucose is considered as an important outcome of photosynthesis, it initiates the cellular respiration in prokaryotes and eukaryotes. Glucose can either help or harm the organisms. It is used by cells in order to make Adenosine triphosphate (ATP) in order to provide energy to the body. Hyperglycemia is cytotoxic which can lead to high inflammation in the body. When there is less glucose molecule in the body the condition of Hyperglycemia exists which can be harmful and sometimes may lead to death (Paine, Pithawalla & Naworal, 2019).
There are certain ways bases which the body can keep a check on the changing levels of glucose molecules and other mechanisms in order to avoid any harmful situations. Diabetes occurs as the body is unable to regulate the glucose molecules.
How glucose molecules regulate?
During eating, the carbohydrate of the food breaks down in the form of simple sugar. These simple sugars can be easily absorbed in the blood through the digestive system leading to higher level of glucose in the blood (Barclay, Cooper, Ginic-Markovic & Petrovsky, 2010). In this situation pancreas help in detecting the increasing glucose molecules and secrete insulin in response. Insulin helps in regulating and controlling the carbohydrates along with checking the fat metabolism.
When the insulin is released in the blood it directs the liver cells, muscle of skeletal and fat tissue to suck the glucose molecules present in the blood. When there is a decrease in the glucose level and it reaches a safe point then secretion of insulin from the pancreas stop.
Hyperglycemia cannot be felt by a person so a person is never in a position to state or feel about his glucose level being up or down. In certain cases there may be a feeling of being very thirsty or being very hungry or a person may urinate more frequently or even become unconscious. A quick hyperglycemia may lead to a serious situation for diabetic people who remain on insulin (Paine, Pithawalla & Naworal, 2019).
There are situations where without eating for a while, there can be a drop in the glucose level present in the blood. Pancreas gets into action when such situation arises by releasing glucagon which is a different molecule. Glucagon helps the liver to change glycogen to glucose in the blood which is a starchy like glucose. The release of glucagon stops as soon as the glucose level in the blood reaches back to a safe position.
Insulin and glucagon work oppositely in order to maintain a reasonable glucose level but there is a co-ordination among them when it comes to regulating the glucose level in the blood. Hyperglycemia may have some simple or complicated symptoms like feeling unwell or become unconscious, damage to the brain or the occurrence of death (Roder, Wu, Liu and Han, 2016). Other effects and symptoms include kidney damage, damage to heart, damage to eye or nerve or some damage to the feet or hands. These complications can take time to develop once there is occurrence of hyperglycemia. The below diagram helps in understanding the regulation of glucose molecules:
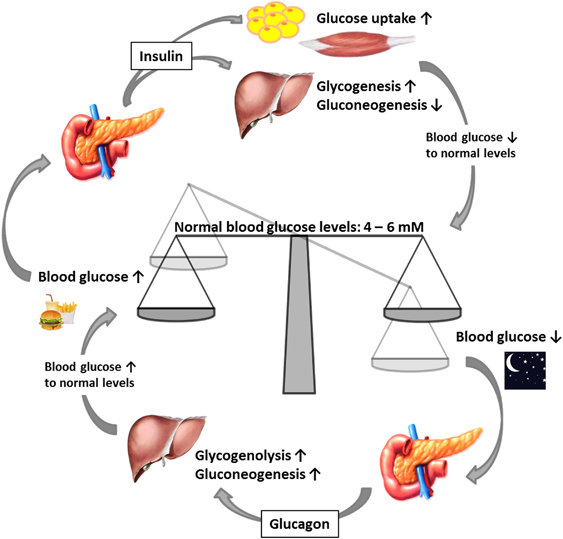
Source: (Roder, Wu, Liu and Han, 2016)
Structure of glucose
There are 6 carbon atoms present in glucose (C6H1206) along with an aldehyde group which is also known as aldohexose. The existence of glucose molecules can be in the form of open chain or in the form of a ring. The ring is formed as a result of intra molecular reaction taking place in the aldehyde C atom and C-5 hydroxyl group to take the form of an intra-molecular hemiacetal. Both remain in equilibrium when present in water and there is a predominance of cyclic one when it reaches Ph7 (Paine, Pithawalla & Naworal, 2019). There are 5 carbon atoms and one atom of oxygen present in the ring which looks like a pyran. Glucopyranose is another name of cyclic glucose molecule. The carbon in the ring is linked with a side group of hydroxyl eliminating the 5th atom which again links back to a 6th atom of carbon which is placed outside the ring in turn forming a group, CH2OH. The below image helps to understand the structure of cyclic and acyclic glucose:
Source: (Barclay, Cooper, Ginic-Markovic and Petrovsky, 2010)
Production of glucose
Glucose can be produced naturally as well as commercially. The natural process may take the form of a product of photosynthesis which takes place in plants and in some prokaryotes. Glucose in animals and in fungi takes place due to the breaking of glycogen and the process is referred to as glycogenolysis (Barclay, Cooper, Ginic-Markovic and Petrovsky, 2010). The break down in plants takes the form of starch. Glucose gets synthesized in the animals in its liver and kidneys.
The commercial process may include production of glucose through starch in the form of enzymatic hydrolysis. There are certain crops which can be a great origin of starch like maize, potato, rice, etc. These crops are used widely in order to produce glucose molecules for e.g. cornstarch is widely used in USA . There are two stages of enzymatic process. In one or two hours at 100 ?C the enzymes starts to hydrolyze the starch into small carbohydrates which contains glucose molecules in a unit of five to ten. In some cases the process may heat the mixture of starch to 130 ?C or more (Zhang & Bar-Peled, 2019). The heating helps to dilute the starch in the water but it also leads to deactivation of enzymes and more fresh enzymes needs to be added after each and every heating.
During the second stage which is referred to as saccharification, the glucose which is partially hydrolyzed gets complete hydrolyzed into glucose molecules with the usage of glucoamylase enzyme which is accumulated from fungus named aspergillus niger. The required condition for reaction are Ph4.0-4.5 and at 60 ?C along with a concentration of carbohydrate weighing 30-35%. Staying in this condition, after fourteen days, the starch will convert in the form of glucose with 96% yield (Rensburg & Ende, 2018). More glucose molecules can also be converted through this process but it will require a higher amount of diluted solution which may not be economical. The glucose solution which is obtained through this process is purified through filters and kept in an evaporator. A solid glucose is formed after several crystallizations.
Functions of Glucose
Glucose is widely used in order to evolve the ecosystem and the metabolism. Glucose has a lesser tendency to react with some proteins existing in amino group as compared to some other hexose sugars. There are many enzymes whose function gets destroyed or reduced due to the reaction referred to as glycation (Dienel, 2018). Many complications related to acute diabetes like blindness, failure of kidney, etc. are probable cause of proteins generated through glycation whereas glucose protein added through regulation of enzymes may be may an essential function.
Source of Energy
Glucose molecules are a great source of energy in almost all the organisms be it bacteria or humans. There are some body cells which fully depend upon glucose while generating energy. The glucose can be used for respiration through aerobic or anaerobic. Carbohydrates are a key source of human energy generated through aerobic respiration and it provides food energy which is at least four kilo calories per gram. Glucose through glycolysis and coming in contact with citric acid cycle is oxidized to form CO2 and water (Dienel, 2018). It generates energy in the form of Adenosine triphosphate (ATP). Insulin with the help of other mechanisms regulates the glucose concentration in the blood.
Glucose contained in the glycolysis
Cells use glucose to exert energy through aerobic or anaerobic respiration. The process starts at initial stages of glycolysis and the very first point of this process is phosphorylation of glucose with the help of hexokinase enzyme to break later and provide energy. There is an instant phosphorylation of glucose with the help of hexokinase enzyme in order to limit the diffusion which can be caused outside the cell.
As a helper
Glucose is an important element which helps in the making of proteins and lipid metabolism. It also helps in the production of vitamin C in some plants and animals. Glycolysis helps to modify the glucose for further usage. Glucose molecules also help in the synthesis process of certain substances like starch, cellulose, glycogen, etc. Lactose present in milk is also one of the forms of glucose (Dienel, 2018).
As a source of absorption
Glucose is contained in dietary carbohydrates in the form of building blocks, in starch, in glycogen or with a combination of another monosaccharide. Glucose also goes straight to provide fuel to brain cells and erythrocytes (Rensburg & Ende, 2018). Some of them reaches the liver and to the muscles and is stored as glycogen. It also reaches the fat cells and is stored in the form of fat. Glycogen is an energy source for the body which is tapped and changed back to form glucose when the body requires some energy.
Quick facts about glucose
Glucose has been derived from a French word glukus which means sweet. Ose in the term glucose molecule indicates that it is a carbohydrate.
It has six carbon atoms and is categorized as a hexose. It can be found in linear or in a cyclic form.
It helps in the supply of energy to the human brain and is important for red blood cells and the cells of muscles. It can also be dissolved in the water.
It is the plentiful monosaccharide available around us which acts as a source of energy for organisms present on the earth. In contains in the plants in the form of sugar which is produced in the course of photosynthesis.
Glucose also forms isomers and they are chemically similar but are different in conformations. D-glucose is naturally processed whereas L-glucose can be processed synthetically.
C6H12O6 is the formula of glucose molecule and in simple form it may also be referred to as CH2O.
References
Barclay, T.G., Cooper, P.D., Ginic-Markovic , M & Petrovsky, N. (2010) Inulin - A versatile polysaccharide with multiple pharmaceutical and food chemical uses. Journal of Excipients and Food Chemicals, 1(3).
Dienel, G.A. (2018) Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev, 99(1).
Paine, J.B., Pithawalla, Y.B & Naworal, J.D. (2019) Carbohydrate pyrolysis mechanisms from isotopic labeling. Part 5. The pyrolysis of D-glucose: The origin of the light gases from the D-glucose molecule. Journal of Analytical and Applied Pyrolysis, 138.
Rensburg, H.C.J & Ende, W.V. (2018) UDP-Glucose: A Potential Signaling Molecule in Plants? Front. Plant Sci.
Roder, P.V., Wu, B., Liu, Y & Han, W. (2016) Pancreatic regulation of glucose molecule homeostasis. Experimental & Molecular Medicine, 48, e219.
Zhang, J & Bar-Peled, L. (2019) How Sweet It Is: Small-Molecule Inhibitors of mTORC1 Glucose Sensing. Cell Chemical Biology, 26(9).