An Overview Of The Properties And Molar Mass Of Ethanol

Ethanol
This research paper will explore the molar mass of ethanol to understand its properties and components. This If looked down on the origin of the word alcohol, it was derived from a word from the Arabic language that was spelled as al-kuhul. At that period, this word signified the subtle and refined particles of antimony that were obtained by the process of distilling which eventually was used as an ingredient for eye makeup. In the ancient period, processed powders were termed as al-kuhul which later on was generally used to signify all the products, which were distilled.
It is by the biological fermentation of several organic materials by which the clear and purest form of liquid alcohol, ethanol is created. Although the ethanol is used by humans widely as an intoxicating drink, in the current scenario, it has widely gained prominence as a biofuel. The process of manufacturing ethanol is very similar to that of the methodology used in beer brewing. It is after being mixed with the petrol that the ethanol is used as a fuel in vehicles or other types of machinery. Studies have made it clear that the use of ethanol as a biofuel would increase the efficiency of types of machinery and would heavily contribute towards the cause of reducing air pollution.
Composition of Ethanol
As mentioned earlier in this article on the molar mass of ethanol, the liquid form of ethanol is generated by the biological fermentation of various organic materials. The agricultural products like corn, sugar cane, wood, barley, and wheat would come under the classification of these organic materials. Since the agricultural products like wheat, contains a high amount of carbohydrates, they show high yield in ethanol production. Thus, wheat is used widely by Canadians for ethanol manufacture.
The organic raw materials which are derived from the agricultural products having a very small amount of value are widely used for the production of ethanol. The products like barley, feed wheat, and corn come under this category. The food crops which sometimes get perished due to natural causes and are of no use for humans or the live stocks could be used for the production of ethanol. If taken the production of ethanol on the worldwide perspective, starch-based crops and corn are widely used for the large-scale production of ethanol. Though there is a hidden risk of abnormal increase in the price of food crops if the demand for ethanol increase in the global market. Hence, scientists are looking for more convenient and less impacting raw materials for ethanol. The researchers have observed that the ethanol could also be produced efficiently from domestic cellulosic biomass feedstocks and other wastes and residues from the agricultural area, forest area, industrial and city wastes, etc.
Implications of Ethanol
- Using as a fuel: In the early period, ethanol was only considered as an intoxicating drink which was often mixed in other beverage products like wine and beer to increase its efficiency to intoxicate. But by the current innovations in science and automobile technology, ethanol has increased its significance as a substitute for the automobile fuels. This would decrease the depletion of hydrocarbon fuels in the world and the pollution created by the combustion of it. The types of machinery that consume the majority of the crude oil products like vehicles could be operated on ethanol and hence could contribute heavily towards the cause of reducing air pollution and global warming. Only a little modification in the air intake of the machine should be made to make it compatible with the ethanol intake. Although the ethanol used to operate the vehicles are termed as gasohol. The gasohol is prepared by mixing the gasoline with the ethanol in the ratio of 9 : 1. Heavy combustion in the engine could by the use of gasohol since ethanol possesses higher saturation of the oxygen and the octane value ranging from 2.5 to 3 which is much higher than that of the gasoline. Unlike gasoline, ethanol is the renewable form of energy and hence would impact the environment heavily.
- Scope of Ethanol as Fuel Cells: If analyzed the evolution of fuel cells in this article on the molar mass of ethanol, it is considered to be one of the best ways to enhance the efficiency of the machines and thus consequently reduce the pollution emission of the vehicles. The use of fuel cells would also help in reducing the dependency on the nations who produce and supply fuel to other countries. The technology of the fuel cells would be sufficient in providing adequate energy for the automobiles in a country. The vehicles which have been manufactured by installing the fuel cell technology along with it have a higher efficiency up to the level of 80 mpg which is way higher than the conventional vehicles. Although the difficulty in containing or storing the hydrogen has been always a humungous change before the manufacturers. This is why the vehicles with the fuel cells are still not successful on a commercial basis. Though if there is substantial progress in the technology, the installation of the fuel cell technology would eventually bring down the level of air pollution to its minimum. In many areas of the world, fuel cell vehicles are used on a large scale to avoid the negative impact on the environment. The inconsiderate use of fossil fuels and other non-renewable resources has increased the perils and extent of global warming. The use of ethanol has also brought a push to make automobiles more eco-friendly. The use of ethanol in the vehicles would decrease the emission of carbon monoxide while the spark ignition than in the case of using gasoline.
- Existence of Co-Products: Since the production of ethanol is a chemical process, there would obviously be a byproduct. If looked at the process of fermenting the grains, around two-thirds of the mass is converted into ethanol. The remaining one-third of the product is very good food for the live stocks since it is very rich in protein content. This food is more suitable for the animals who are ruminating in nature which include, cows, buffaloes, sheep, goats, etc. If said scientifically, this byproduct is termed as Distillers’ Dried Grains or in abbreviation DDGS. In the farms of Canada, this DDGS is used as the cattle feed which has displayed high productivity and accelerated cattle growth. Not ending here. The waste product from the live stocks after eating this DDGS is excellent manure for agriculture.
Moving on from the engines which only used gasohol with 9 : 1 ratio of gasoline and ethanol, the American companies have created E 85 engines which use the fuels with 85 % of ethanol with remaining 15 % of gasoline. The number of E 85 vehicles is increasing on the American roads day by day and they have the versatility to use pure gasoline and the gasoline which contains 85% ethanol. Unlike the gasoline, ethanol is a fuel which is devoid of any water content and hence the E 85 engines could be operated in an environment with very less temperature which go below the level of -40 degree Celsius.
Although the use of the ethanol is made familiar in the near past the first use of it in the automobiles was recorded to be in 1979 though they were not being addressed as the alcohol fuel. It took decades by the automobile companies to check the authenticity and the efficiency of the gasohol. There was a series of researches conducted on other types of machinery too, which included the experiments conducted at the Lake Area Vo Tech at Watertown.
Let us have a look at the modus operandi of the fuel cells. In the vehicles, with fuel cells, the hydrogen and oxygen are combined via a chemical reaction. By this reaction, electrical energy is created in the system which is then used then after to move the wheels of the vehicle. Since there is no combustion happening in the machine, there would be very less emission of pollutants in the atmosphere. If said in a rough manner, the fuel cells actually work as a battery and provide electricity in the circuit. However, the fuel cell is more convenient than an ordinary battery since it continuously provides with energy without any requirement for recharging, since it shows no sign of wearing down.
In the process of the fermentation of the starch, another major byproduct is carbon dioxide. It is heavily producing as a byproduct when the chemical reaction of converting the starch or glucose into ethanol is commenced. The byproducts obtained after the fermentation like fibrotein TM could be used to enhance the nutrient intake of humans and hence could be used as a food additive. The remain corn gluten is an excellent food for the live stocks.
The link between the environment and ethanol production.
There is no need to say it apart in this report on the molar mass of ethanol that the increasing level of industrialization has deteriorated the level of the environment. The increasing level of pollution has accelerated the pace of emission of greenhouse gases which is eventually increasing the intensity of global warming. The gases like methane, carbon dioxide, and nitrous oxide come under the category of greenhouse gases. Because of these gases, the heat entering into the atmosphere of the earth via radiation gets trapped and by this phenomenon, the average temperature of earth atmosphere increases gradually. If the temperature of the environment increases, then the whole balance of the ecology gets interrupted. The whole balance of the climate has been changed because of this and is posing the lifestyle and living beings in various parts of the world. The alteration in the pattern of climate has caused a great loss to the farmer community since they have followed the seasonal farming. The ecosystems existing in the places below the sea level are being destroyed since the ice in the polar areas is melting at a very fast pace. Scientists have predicted that the average temperature of the global atmosphere would rise from the range of 1 to 3.5 degrees Celsius by the year 2100. Consequentially, the average level of the sea would get raised by 50 cm.
- The environmental advantage of using Ethanol as fuel
- Carbon Monoxide: In the vehicles, the carbon monoxide is generated because of the partial combustion of fuel. This case mostly occurs in oxygen-deficient fuels like petrol. Since the molecular structure of ethanol contains extra oxygen, the fuel gets completely combusted in the machine. It had been made clear by the scientists that by the use of ethanol, carbon monoxide emission could be reduced by around 30 %.
- Carbon Dioxide: The emission of carbon dioxide is majorly being contributed by the fuel combustion in the automobiles. It has been observed that if the gasoline is being mixed with 10 % ethanol, the emission of carbon dioxide could be substantially reduced to an extent of 6 to 10 %.
- Nitrous Oxide: To suffice the requirement of the ethanol, farmers and industries may have to come up with a higher production of agricultural crops. The increased production of crops would consequentially increase the emission of nitrous oxide. The increased use of science and technology has actually helped in decreasing the nitrous oxide.
We have now covered most of the general aspects of the ethanol. As mentioned above in this report on the molar mass of the ethanol, ethanol is obtained by the fermentation of biological materials and sustains the characteristics of a volatile liquid. We have not explored the significance of the molar mass of ethanol until in this article. It could be kept in mind that apart from being induced artificially, the process of fermentation occurs naturally in the environment since glucose and fructose are abundantly available in fruits and other crops. Even some of the organisms like Pentailed Treeshaw in ecology seek precisely for the fruits which are fermented. Though because of the sour taste and its pungent smell, fermented fruits are avoided by most of the herbivores in the ecology.
It is a commonly known fact in the discipline of biology that the fermentation of the carbohydrates would result in the production of the ethanol. Since the ethanol could be directly absorbed by the digestive tract of humans, it is used as an intoxicating drink by humans. The ethanol very soon gets assimilated into the human body it suddenly intoxicates the human nervous system. Apart from possessing the intoxicating characteristics, ethanol also has anti depressing nature that would help in easing out the tension and stress felt by human beings. Although it is also notorious for inciting a very aggressive and violent nature among those who drink it. The intoxicating effect would depend directly on the amount of ethanol ingested. The biological condition and the age of the person also determine the level to which the ethanol intoxicate one.
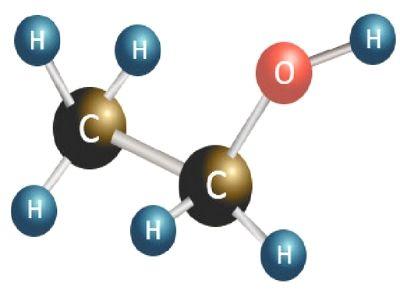
We have talked about the normal life effects of ethanol on people in daily life. If studied about the molecular mass of ethanol, we could understand the element in a very scientific way. Before going further to the elaboration molar mass of ethanol, let us understand the molecular structure of it. The molecular formula of the ethanol could be mentioned as C2H6O. From referring to it, the reader could understand that the ethanol is made up of basically three elements, which are, Carbon, Hydrogen, and Oxygen. By looking at the molecular formula of the ethanol, it could be understood that a single atom of ethanol contains two carbon atoms, six atoms of hydrogen, and one atom of Oxygen. Since the molecule of ethanol contains various types of atoms, they all have different atomic mass. If taken the case of a carbon atom it would be equal to around 12.0107, similarly, the atomic mass of hydrogen is 1.00794. The atomic mass of oxygen would be around 15.9994.
The molecular mass of a molecule is the total sum of the mass held by different atoms in it. The molecular mass of an element or a compound is generally displayed in reference to the atomic mass unit. The concept of the molecular mass of ethanol is very different from that of the molar mass of ethanol. The molecular mass is actually the total mass of a single molecule of the ethanol, although the molar mass of ethanol is the overall mass of the molecules in one mole of the concerned elements. In this context, the mass of the material could be expressed using the unit of g/mol. It has been computed by the scientists that the molar mass of ethanol is 46.864 g/mol.
Let us explore the Characteristics of ethanol further.
It may come across your mind what actually is the scope and significance of ethanol in the scientific and daily life. It is the atomic mass of included elements in a compound that molecular mass depends on. The calculation and the estimation of the molecular should be done very cautiously since you would be dealing with a very low measure of mass. The normal arithmetic operations are not sufficient for the calculation of the molecular mass of ethanol.
While dealing with the ethanol particles, the concerned person should be very cautious since it possesses high inflammable characteristics. This condition should be taken frivolously by the people, since there is a high chance of ethanol to catch fire, in the temperature higher than 29 degree Celsius given that the ethanol is concentrated up to the level of 40 %. Though the chance of ethanol being ignited without the aid of an external source is very low. If you had watched some international culinary food shows, it would have come to your notice that some sort of food gets inflamed for a little span of time. This elegant view is made possible with the help of ethanol.
Although ethanol is being made available by the manufacturers in different concentrations, the molar mass of ethanol never gets affected by it. Although we have mentioned various grades of ethanol in the previous section of this report in the molar mass of ethanol, let us look at some of the general sections or qualities of ethanol.
Denaturized Alcohol – This is the form of the alcohol which is denaturalized using some additional content and hence is not potable If consumed in this form, the denaturized alcohol would cause some biological complications in the person who consumes it. This type of ethanol is generally used for industrial and manufacturing purposes.
Absolute alcohol – In this type of ethanol, the content of water would be to its minimal level possible. It would not be required to mention it specifically that the concentrated form of ethanol is not fit for consumption. The major implication of absolute is done in chemical and biological labs for commencing specified and customized experiments.
Rectified alcohol – Apart from the type of alcohol mentioned above, rectified alcohol is best suited for consumption as an intoxicating beverage. Although this type of alcohol contains 94 % of ethanol in it, several compounds are mixed in it to make it more potable. As per the preferences and taste of the drinker, the concentration of ethanol is adjusted.
Whatever manipulation is done with the concentration or whatever raw material is done to manufacture the ethanol; the molar mass of ethanol remains the same. Apart from using the ethanol compound in its purest form, it is mixed with other compounds to carry out the intended activity. Ethylene is identified as one of the most prominent and significantly used chemicals in the industrial area. The compound to artificially ripen the fruits are created using ethanol and hence the compound has a higher scope in the agricultural field. Although the derived compound ethylene has a negative impact on the environment. In the compound of ethylene, the molar mass of ethanol could not be taken into account, since it has been changed into another compound. As per the content discussed in this article on the molar mass of ethanol, ethanol holds great significance in both the daily life and industrial field. It has been made clear that the molar mass of ethanol plays a very crucial role in the inherent nature of the element.