Explore The Characteristics Of Enzymes And Their Functions

What are enzymes?
Enzymes are peculiar proteins essential in furthering and encouraging biochemical reactions in living organisms. The catalyst characteristics of enzymes speed up the process of chemical reactions without being permanently altered or consumed. It comprises different biological processes, such as digestion, DNA replication, metabolism, and cellular signaling.
With the aid of enzymes, vital metabolic reactions occur at compatible rates within a body. Enzymes facilitate lowering activation needed to produce energy for a reaction, making it efficient and favorable.
Enzymes play a vital role in controlling and regulating biological processes. As a consequence of cellular signals, they can be inhabited or activated, making sure that the biochemical reactions occur at the correct time and quantity.
What are the characteristics of enzymes? The regulation, thereby, leads to maintaining homeostasis, reciprocating to environmental changes, and performing critical functions like growth, defense mechanism, and reproduction. In addition, enzymes demonstrate exceptional specificity, allowing them to do particular tasks within the complex network of biochemical reactions.
In this blog on the characteristics of enzymes, we will now dig into several other vital details concerning enzymes.
The structural feature of enzymes
What are the characteristics of enzymes? Enzymes have a hierarchical structure comprising varied levels such as primary, secondary, tertiary, and quaternary. Different levels of structure in the enzymes add to their complete shape and functionality.
Primary structure: Enzymes are proteins; an enzyme’s primary structure relates to its amino acid sequence. The primary structure of enzymes is formed by the amino acid sequence connected jointly by peptide bonds.
The particular placement of amino acids in the primary structure is distinctive to each enzyme and is concealed by the genetic details in the organism’s DNA.
Secondary structure: It talks about the local folding patterns rising from interactions between amino acids. Alpha helix and beta-sheet are general types of secondary structures in enzymes.
A beta-sheet comprises the polypeptide chain shaping in a sheet-like structure through hydrogen binding between adjacent strands. In contrast, in the alpha helix, the polypeptide chain curls into a helical structure fixed by hydrogen binding between amino acids.
Tertiary structure: It relates to the complete 3D conformation of the enzyme. It is established by the interplay between amino acids far away in the primary structure. The functioning of the tertiary structure is based on the folding of an enzyme in its 3D conformation.
What are the characteristics of enzymes? Inaccurate folding, due to genetic mutation or external factors, can give rise to misfolding diseases or enzymatic activity. The specific folding process is done with the help of chaperone proteins and molecular chaperones that assist enzymes in getting their right conformation.
Quaternary structure: Enzymes in certain situations may have a quaternary structure, which connects several polypeptide chains or subunits. Different interactions balance the quaternary structure, such as ionic bonds, hydrogen bonds, and hydrophobic interactions between subunits.
The structure is essential for specific enzymes to function perfectly, as it may give enhanced stability, building active sites comprising residues from separate subunits or allosteric regulation.
Substrate specificity of enzymes
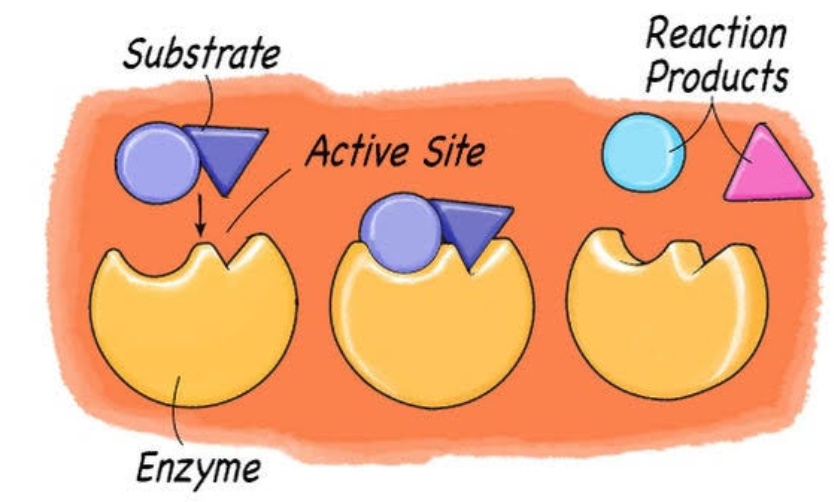
The enzyme-substrate specificity deals with the potential of enzymes to bind and catalyze reactions with particular selective substrates. Enzymes display exceptional specificity, ensuring they interact with selective substrates while removing others. Such specificity is vital for adequately functioning cellular processes and biochemical pathways.
- Enzyme-substrate specificity model: Two models describe the enzyme-substrate specificity. The blog on characteristics of enzymes discusses the models in the below part.
Induced fit model- It gives a more positive and correct portrayal of enzyme-substrate specificity. As per the model, there is no rigidness in the active site of an enzyme; instead, it is flexible and goes through conformational changes upon substrate binding.
The binding of the substrate produces a conformational change in the enzyme leading to a more optimal fit between substrate and enzyme. The model lays stress on the powerful interaction between substrates and enzymes.
Lock and key model- An old notion is used to describe enzyme-substrate specificity. As per the model, there is rigidity in the shape of the active site that correctly meets the shape of the substrate. Hence, the name lock and key fit together.
What are the characteristics of enzymes? Here, the key is the substrate that specifically fits the enzyme’s active site. The model states that the active site is carried out and does not alter its conformation upon substrate binding.
- Active site: It is the particular region where the substrate binds, leading to the occurrence of a catalytic reaction. It is mainly an aperture or a small pocket within the enzyme’s structure. The active site has a distinct shape, charge distribution, and chemical environment corresponding to the substrate. The complementary nature permits specific interaction between the substrate and the enzyme, like ionic and hydrophobic interactions, hydrogen bonding, etc. The placement of the active site can change as per the difference in enzymes. The active site in some enzymes is located in pockets or deep crevices; in the rest, it may be at higher exposed surface regions. The active site typically comprises amino acid residues generated from different parts of the polypeptide chain, jointly brought through the folding of the proteins.
- Enzyme-substrate complex: It relates to the temporary link established between an enzyme and its substrate in the catalytic reaction process. On entering the active site of an enzyme, the substrate goes through specific interactions, like hydrophobic interactions, electrostatic interactions, and hydrogen bonding, with the residue of amino acid present in the active site. The process of binding gives rise to the building of an enzyme-substrate complex. The complex permits the enzyme to assist in changing the substrate into products with the aid of a catalyst. The products are sent after the reaction is finished, and the enzyme is free to bond to different substrate molecules.
How do enzymes help in substrate binding and catalysis?
The role of enzymes in substrate binding and catalysis is crucial as it helps in the occurrence of chemical reactions in biological systems. Enzymes increase the rate of reactions by reducing the activation energy needed to change substrates into products.
Enzymes perform like catalysts by giving an optional reaction pathway that needs less energy to begin the reaction. It is gained by reducing the activation energy, which is the energy obstacle that must be controlled for the occurrence of the reaction.
Activation energy is the lowest energy needed for the occurrence of a chemical reaction. The energy obstacle must be controlled for reactant molecules to meet the changing state and form products.
Enzymes reduce activation energy by giving an optional reaction pathway with a reduced energy obstacle. They regulate the changing state, minimizing the energy required to process the reaction, facilitating the reaction rate.
Kinds of enzyme catalysis
The blog on characteristics of enzymes will discuss the kinds of enzyme catalysis.
Acid-base catalysis: In these characteristics of enzymes, enzymes utilize acidic or basic residue to accept or donate protons at the reaction time. The proton change can help break or form bonds in the substrate, giving rise to a catalyst reaction.
Metal ion catalysis: Enzymes are often bound by metal ions; they can participate in catalytic reactions by being electron donors or acceptors, assisting in redox reactions, or stabilizing charged intermediaries. Catalysis with metal ions is specifically significant in enzymes taking part in redox reactions and metalloenzymes.
Covalent catalysis: It takes part in building a transient covalent bond between the substrate and the enzyme. What are the characteristics of enzymes? The covalent bond balances the transition state and the ensuing delivery of the product. Regular instances include building a covalent enzyme-substrate intermediate or transferring an operational group from the enzyme to the substrate.
Catalysis by strain: Enzymes can cause distortion or strain on the substrate, making it easier for specific bonds to break or form during the reaction. The strain on the structure of the substrate can reduce the activation energy needed for the reaction.
Catalysis by approximation: In this kind of catalysis, two or more substrates are brought by enzymes into proximity, adding to the reaction by enhancing the probability of their clash and minimizing the entropy obstacle. What are the characteristics of enzymes? It is generally seen in enzymes taking part in reactions between two separate substrates.
Regulation of enzymes
Enzyme regulation deals with controlling and modulating enzymatic activity to stabilize biochemical pathways and react to changing cellular conditions. Several mechanisms can regulate enzymes: competitive inhibition, allosteric regulation, non-competitive inhibition, and feedback inhibition. Let us explain each of them in this blog on the characteristics of enzymes.
Competitive inhibition: It takes place when a molecule, called a competitive inhibitor, contends with the substrate for binding to the enzyme’s active site. There is a similarity in shape between the competitor inhibitor and the substrate, and it can bind changeably to the enzyme’s active site, stopping the substrate from binding.
What are the characteristics of enzymes? Such inhibition can be controlled by enhancing the concentration of the substrate, as it permits more substrate molecules to beat the inhibitor and bind to the enzyme.
Allosteric regulation: It binds a regulatory molecule, called an allosteric effector, to the allosteric enzyme site. The binding can increase or inhibit the activity of the enzyme. Allosteric regulation permits fine-tuning and coordinating metabolic pathways in reaction to cellular signals or changes in metabolite concentrations.
Non-competitive inhibitor: It binds the inhibitor on the enzyme site separate from the active site, referred to as the allosteric site. The binding causes a conformational change in the enzyme, minimizing its catalytic activity. Dissimilar to competitive inhibitors, this inhibitor is not affected by changes in the substrate concentration, as the inhibitor can bind together the substrate.
Feedback inhibition: It is also referred to as end-product inhibition and is a regulatory mechanism generally seen in metabolic pathways. In this inhibition, the end product of a pathway acts as an allosteric inhibitor for a previous enzyme present in the pathway.
When the product has a high concentration, it binds to the allosteric site of the enzyme, inhibiting its activity and stopping the excessive production of the product. It assists in keeping homeostasis and stops unwanted energy expenses.
Factors impacting enzyme activity
Many factors can majorly impact the activity of enzymes. Identifying and understanding these factors is essential for optimizing enzymatic reactions and comprehending their behavior in varied conditions. The key factors impacting enzyme activity are temperature, substrate concentration, pH, the existence of cofactors and coenzymes, and enzyme concentration. Let us discuss these in this blog on the characteristics of enzymes.
Temperature: Enzyme activity is greatly impacted by temperature. When the temperature rises, the rate of enzymatic reactions usually rises due to the increased kinetic energy of molecules, giving rise to more clashes between the substrate and enzymes. But increased temperatures can denature enzymes, leading to their three-dimensional structure and activity loss.
Substrate concentration: The substrate concentration impacts the rate of an enzymatic reaction. When the substrate concentration enhances, the reaction rate enhances proportionality until the active sites become saturated with substrates. What are the characteristics of enzymes? During saturation, expanding more substrate will not enhance the reaction rate as all active sites are filled, and the reaction has reached its maximum velocity.
pH: There is optimal pH in enzymes where they depict maximum activity. The pH impacts amino acid residues’ active site ionization states, influencing the enzyme-substrate interaction. Divergence from the optimal pH can derange the enzyme’s structure and catalytic efficiency.
Cofactors and coenzymes: Several enzymes need extra non-protein molecules known as cofactors and coenzymes to function. Cofactors can be inorganic ions, like iron or zinc. Simultaneously, coenzymes are organic molecules, like vitamins or coenzyme A. Cofactors and coenzymes help in catalysis by giving essential chemical groups or changing electrons during the reaction.
Enzyme concentration: The rate of an enzymatic reaction relies on the enzyme concentration. Enhancing the enzyme concentration usually enhances the reaction rate as more active sites are present for substrate binding. But at increased enzyme concentration, the reaction rate may become less by the availability of substrates.
Usage of enzymes in industrial applications
There are wide-ranging applications of enzymes in different industrial sectors because of their exceptional specificity, versatility, and efficiency. A few of the applications of enzymes in different industries are discussed in this blog on the characteristics of enzymes.
Food industry: Enzymes have a vital role in food processing and production. It is utilized to enhance the texture of the food product, flavor, and nutritional value. For instance, amylases are used in baking to pull down starches into sugar, whereas proteases assist in tendering meat.
What are the characteristics of enzymes? Rennet is an enzyme used in cheese production, and pectinases help clarify fruit juice. For fermentation, several beverages are produced with enzymes, like brewing and wine making.
Biotechnology and pharmaceutical: Enzymes in the biotechnological and pharmaceutical sectors produce therapeutic proteins, like vaccines and insulin. Enzymes play a crucial role in DNA sequencing, which is also helpful in gene expression and molecular cloning methods. What are the characteristics of enzymes? They are used in enzymatic assays, diagnostics, and drug synthesis.
Environmental application: There is a huge contribution of enzymes in environmental applications. They are utilized in treating wastewater to pull down organic matter and pollutants. What are the characteristics of enzymes? Enzymes such as hemicelluloses and cellulases produce biofuels from plant biomass.
They are also utilized in bioremediation, which uses natural biological processes to reduce pollutants in soil and water. It is crucial in composting and breaking down organic waste, handling waste, and minimizing environmental impact.
Concluding thoughts
Enzymes are crucial for biological processes. Not having them, the processes of life will become slow and sluggish. Enzymes are particular for specific substrates and have different characteristics. The characteristics of enzymes make them useful for industrial applications.
Their specificity permits selective reactions, reducing unwanted byproducts. They work under lower reaction conditions, minimizing energy consumption and environmental impact.
Frequently asked questions
What are the main characteristics of enzymes?
The main characteristics of enzymes include being proteins, exhibiting specificity, acting as catalysts, and being regulated in their activity.
Examine the role of temperature in influencing the activity of enzymes.
Temperature impacts the activity of enzymes by enhancing reaction rates to an optimal temperature, after which the increased temperatures can denature enzymes and minimize their activity.
Total Assignment Help
In case, you are looking for an opportunity to work from home and earn big money. TotalAssignmenthelp Affiliate program is the best choice for you.
Do Visit: https://www.totalassignment.com/affiliate-program for more details
Total Assignment Help is an assignment help Online service available in 9 countries. Our local operations span Australia, the US, the UK, Southeast Asia, and the Middle East. With extensive experience in academic writing, Total Assignment Help has a strong track record of delivering quality writing at a nominal price that meets the unique needs of students in our local markets. We have a specialized network of highly trained writers, who can provide the best possible assignment help solution for all your needs. Next time you are looking for assignment help, make sure to give us a try.



